A leak may be considered a ‘chink’ through which gas leaks, in a system understood to be hermetic. In terms of dimensions, a leak is quantified by indication of the quantity of gas (expressed in volume) leaking, with a given ΔP at the sides of the leak, in one second. A gas flow takes place in a channel if, between the channel’s extremities, a pressure difference is maintained. The capacity of a channel to enable passage of the gas flow depends not only on the pressure difference between the two extremities of the channel but also on the channel’s geometric characteristics. A magnitude (conductance) is therefore adopted. This is defined as the relation between the gas flow and the pressure difference between the extremities of the channel: C = Q / (p1 - p2) expressed as m3/s or l/s.
- The ideal gases equation finds expression in the following formula:
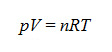
where n is the number of moles of gas contained in volume V at pressure p and absolute temperature (Kelvin degrees [K]), and R is the gases constant (8.31441 j·mol-1·K-1). In this manner, if the pressure is a constant of the system, the equation is obtained, with the flow dependent upon gas volume variation, on pressure and on time:
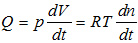
The unit of measurement for the leakrate (Q) according to the International System (SI) is Pa·m3/s. The mbar·l/s unit is a permissible unit of measurement of flow, according to the SI, since it is linked to the former unit of measurement by multiples.
1 mbar·l/s = 10-1 Pa·m3/s (1 Pa = 10-2 mbar and 1 m3 = 103 litres).
In the case of mbarL/s units, the leakrate is Q= 1 mbar·l·s-1 if, in a closed and evacuated chamber of a volume of 1 litre, the pressure increases, in 1 second, by 1 mbar, or, in the case of chamber overpressure, decreases by 1 mbar.
However, it is not always possible to have the leak value expressed in international system units of measurement. It is therefore appropriate to convert these values into Pa·m3/s or mbar·l/s.
A specific instance is to be found in the coolants sector, where coolant leak values are expressed as g/year. Mass flow in this case is transformed into g/Mole and then Moles/s so it is finally transformed into gaseous flow. A table is provided below in which conversion from mass flow to equivalent flow is applied, specifying conversion of g/year values of the key coolants and other gases into the equivalent nominal flows of helium in mbar·l/s.
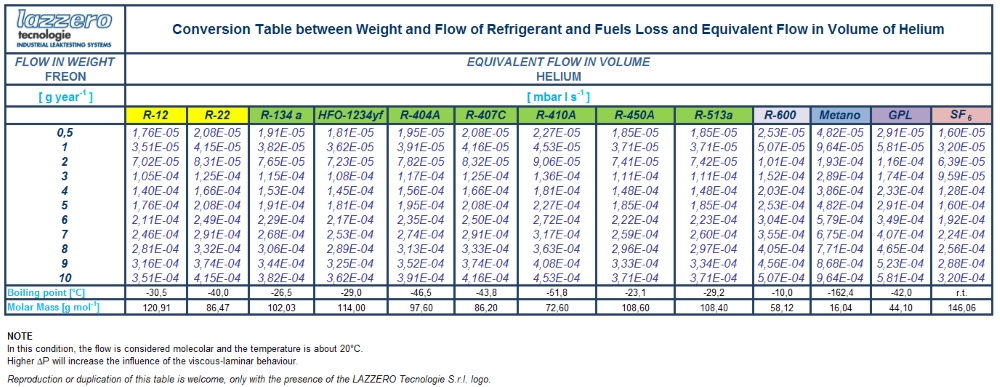
The transformation from the stream by weight (g / year) equivalent flow of helium (mbar·l/s) can be simply achieved by multiplying g / year for the following factors: