Conversion is required in order to compare the equivalent flow generated by a leak specified for a gas which is not the gas measured. However, beforehand, one must be familiar with, or one must determine the flow regime to be considered when performing the transformation. In the laminar flow, due to involvement of the molecular set, we note a clear dependence upon the dynamic viscosity of the gas used. If the value generated by a leak is known, the equivalent can be calculated in another gas (the dynamic viscosity of which is known) by means of the established formula, given below:
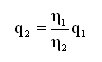
This formula applies when laminar flow conditions obtain.
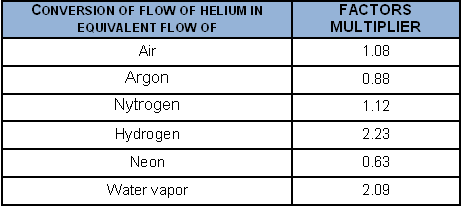
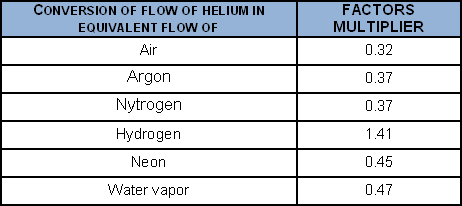
To convert a helium flow into the flow of certain gases, when the laminar regime obtains:
In the presence of molecular flow conditions, the molecules move independently the one from the other; thus, instead of the motion of the gas as a set, one must consider the motion of the single molecules.
We therefore obtain the following expression, dependent upon the molar mass of the gases used:
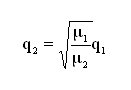
To convert a helium flow into the flow of certain gases, when the molecular regime obtains:
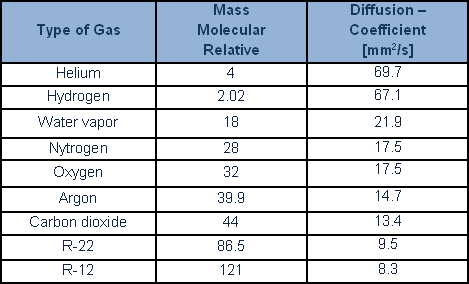
For leakrate values of more than 10-2 mbarL/s, it is still possible to determine a turbulent flow regime. This flow characterises big leaks and marked pressure difference. Simple calculation which considers a specific geometric conductance indicates, according to the literature, that flows of less than 10-6, and, occasionally, of less than 10-7 mbar·L/s, are to be considered molecular, while flows of more than 10-4 mbar·L/are to be considered laminar. However, in practice, real leaks are never, in practical terms, characterised by a single, geometrically defined, leak channel. Laboratory experimentation shows that the behaviour of leaks with flows of less than 10-4 mbar·L/s (occasionally even 10-3 mbar·L/s) conforms to the laws governing molecular as opposed to viscous flow. This happens because the leak flow is the product of a sum of parallel channels (‘chinks’) in which the flow is much smaller, and most surely molecular. An important property of gases is also well worth considering: diffusion of one gas into another gas. Diffusion of lighter gases is more rapid, conditions being equal, than for heavy gases. According to Graham’s law, the diffusion of gases is inversely proportionate to their relative molecular mass.
A number of diffusion values are provided, for the key gases and for coolants:
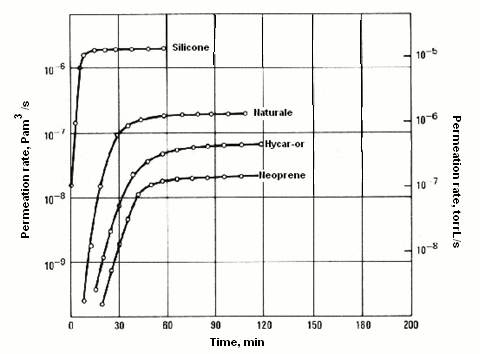
Another property is permeation, understood as the passage of a fluid within and outside a solid barrier with no apertures. This process entails diffusion through a solid, and it may also call into play many phenomena, such as adsorption, dissociation, migration and desorption. Permeation may adversely impact leak detection using helium in cases of small leaks and of prolonged test time. One material worthy of special attention is the Teflon used for packings. Helium readily permeates through Teflon. The graph provided below features a number of curves displaying the permeation rate of helium at various pressures through rubber packings.
The thickness considered is 4 mm with 4-mm cross-section over a length of 25 mm at 25°C (Pam3/s).